Atomic Structure Tutorial for Grade 10 Students
About Course
Atomic Structure for Grade 10 Students
Welcome to the “Atomic Structure” course, specifically designed for Grade 10 students. This course provides an in-depth exploration of the fundamental building blocks of matter—atoms. Through engaging content, interactive elements, and practical exercises, students will gain a thorough understanding of atomic structure and its significance in the field of science.
Course Objectives:
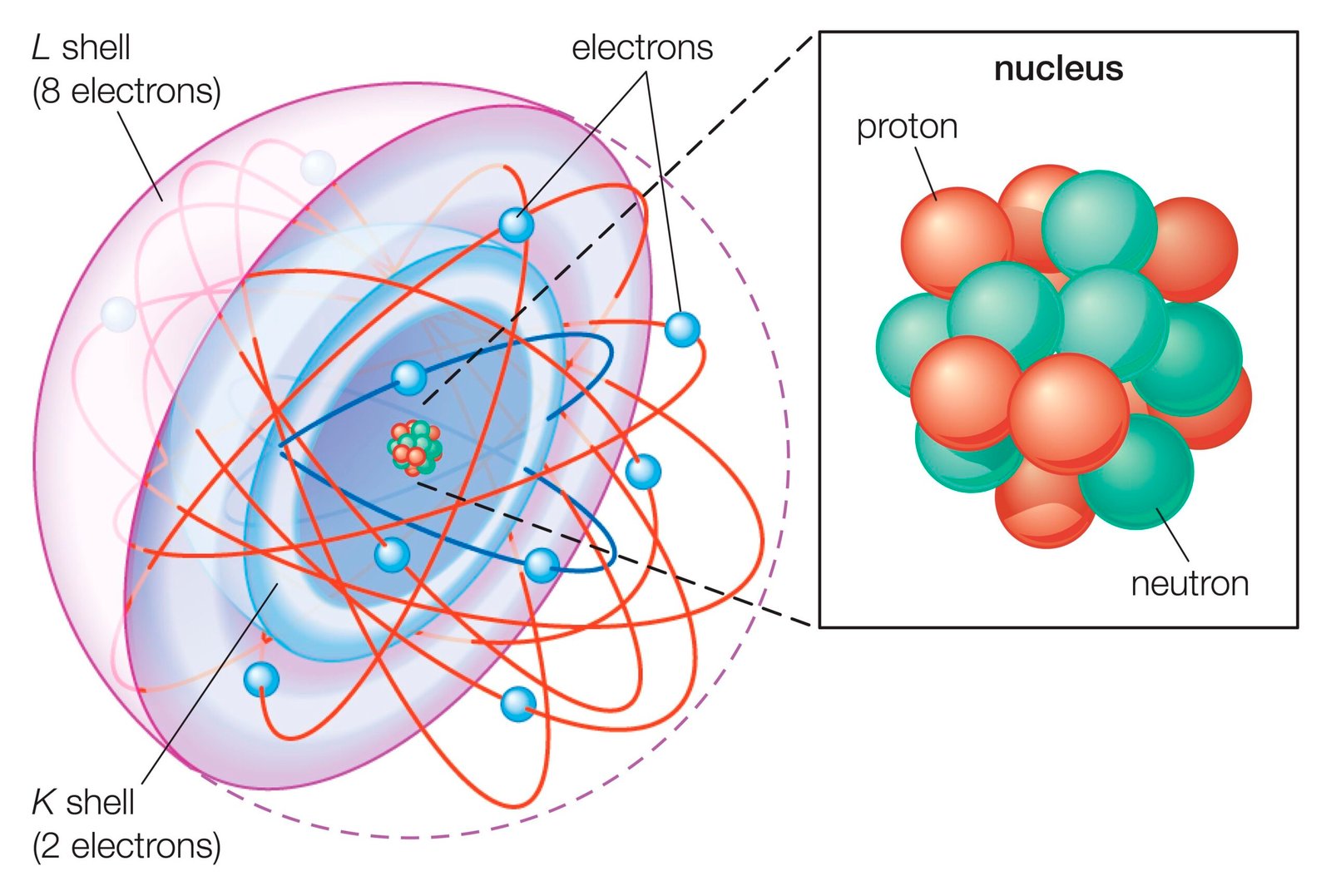
– Understand the Basics of Atoms: Learn about the definition and importance of atoms in the composition of matter.
– Identify Subatomic Particles: Recognize and describe the roles of protons, neutrons, and electrons in an atom.
– Explore Atomic Number and Mass Number: Understand how these numbers define elements and their properties.
– Differentiate Isotopes: Learn about isotopes and their unique characteristics.
– Practice Electron Configuration: Understand how electrons are arranged in an atom and practice determining electron configurations.
Course Features:
– Interactive Elements: Utilize online simulations and quizzes to visualize and practice atomic structures.
– Real-life Examples: Connect atomic theory to real-world applications in various scientific fields.
– Engaging Exercises: Participate in hands-on activities to reinforce learning and understanding.
Why Take This Course?
Understanding atomic structure is a crucial foundation for further studies in chemistry, physics, and other scientific disciplines. This course aims to make learning about atoms exciting and accessible, preparing students for more advanced topics in science.
Join us on this fascinating journey into the microscopic world of atoms and unlock the mysteries of matter!
Course Content
Atomic Structure
-
Introduction
00:00
What is an Atom?
2. Subatomic Particles
3. Atomic Number and Mass Number
4. Isotopes
5. Electron Configuration
Student Ratings & Reviews
